First Use of Speedboat® UltraSlim in Latin America
Our latest device, Speedboat® UltraSlim, has now been successfully used in Latin America for the first time.
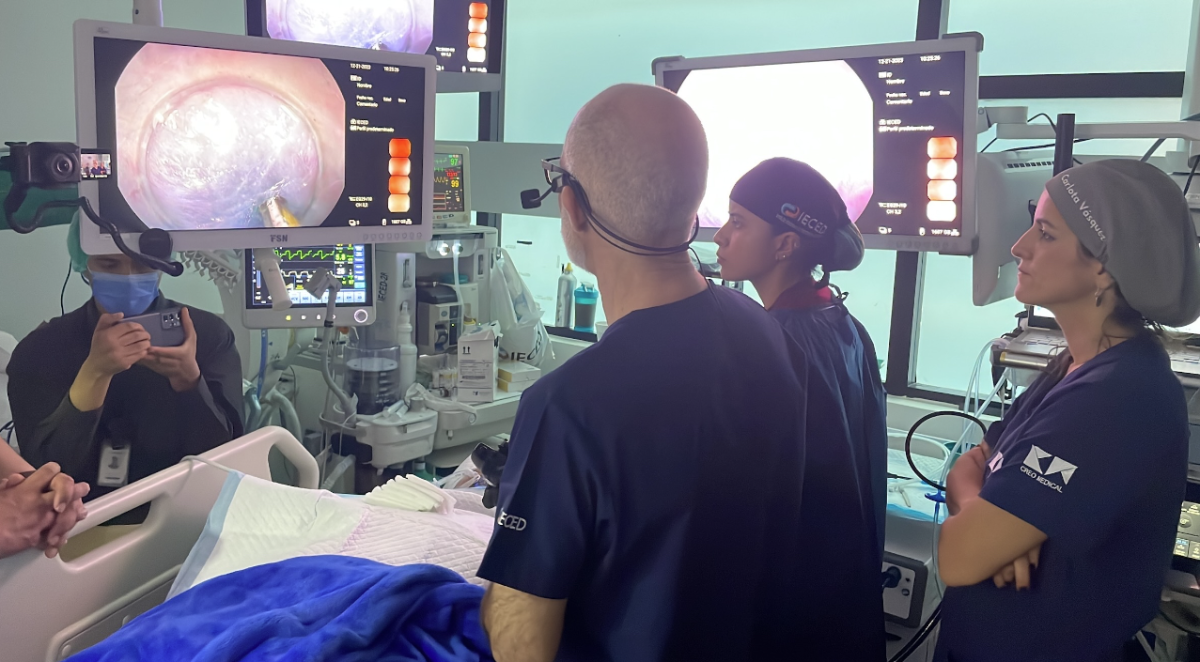
Speedboat® UltraSlim was used at the Ecuadorian Institute of Digestive Diseases (“IECED”) in late December, where Dr Carlos Robles Medranda, Dr Michel Kahaleh and Dr Eduardo Albéniz, supported by the Creo clinical team, performed 12 procedures using Speedboat® UltraSlim over three days, as well as performing the first ever F-POEM procedure with the device. F-POEM (Endoscopic Fundoplication Peroral Endoscopic Myotomy) is a minimally invasive endoscopic procedure used to treat oesophageal motility disorders in patients who are prone to gastroesophageal reflux.
Following the limited market release of Speedboat® UltraSlim in December 2023 the device has been successfully used in multiple clinical applications and was used in the UK, USA to treat precancerous lesions in the colon, oesophagus and stomach, in addition to performing oesophageal and gastric POEM procedures (a procedure which addresses swallowing disorders and gastroparesis).
Carlos Robles-Medranda, Head of the Endoscopy Service at IECED, said:
"I had the privilege of hosting these distinguished physicians, facilitating the successful execution of 12 procedures over three days using Speedboat UltraSlim. The excitement around the device was noticeable, and we eagerly anticipate the positive impact it will have on enhancing our patient pathways as we look to address an increased number of cases."
Dr Michel Kahaleh, who performed many of these procedures at IECED, commented:
"From the very first time when I saw the device, I knew that it was born to do POEM procedures. It allowed me to glide over the muscularis when tunnelling. Once you have acquired the knowledge to use this technology, you’re really going to save procedure time. The technology allows the endoscopist to complete an F-POEM procedure with a single device, whereas previously it required three different devices."
Craig Gulliford, Chief Executive Officer of Creo Medical, said:
"The procedures performed at IECED have shown the full potential of the new Speedboat UltraSlim. We were thrilled to witness the successful execution of the F-POEM procedure using solely the Speedboat device, a task that traditionally requires three separate instruments. This not only showcases the device's efficiency but also enhances the cost-effectiveness of the procedure, underscoring the economic advantages Creo’s technology can deliver to our valued customers."
Posted 10/01/2024
For press enquiries please contact media@creomedical.com. For all other enquiries please visit our Contact page.

