First European Speedboat® UltraSlim procedure marks early commercial launch
Speedboat® UltraSlim has moved into early commercial launch. Following the completion of the design transfer to manufacturing, the first procedure using the device in the UK was performed earlier this week.
This first use of Speedboat® UltraSlim was for a lower Gastrointestinal (“GI”) tract procedure, and comes shortly after the Company announced the UK and EU regulatory pathway had been accelerated by approximately 18 months. The Company expects the first European upper GI cases to follow soon, as well as first upper and lower GI cases in the USA, following the 510(k) clearance being received from the US Food & Drug Administration in November 2023.
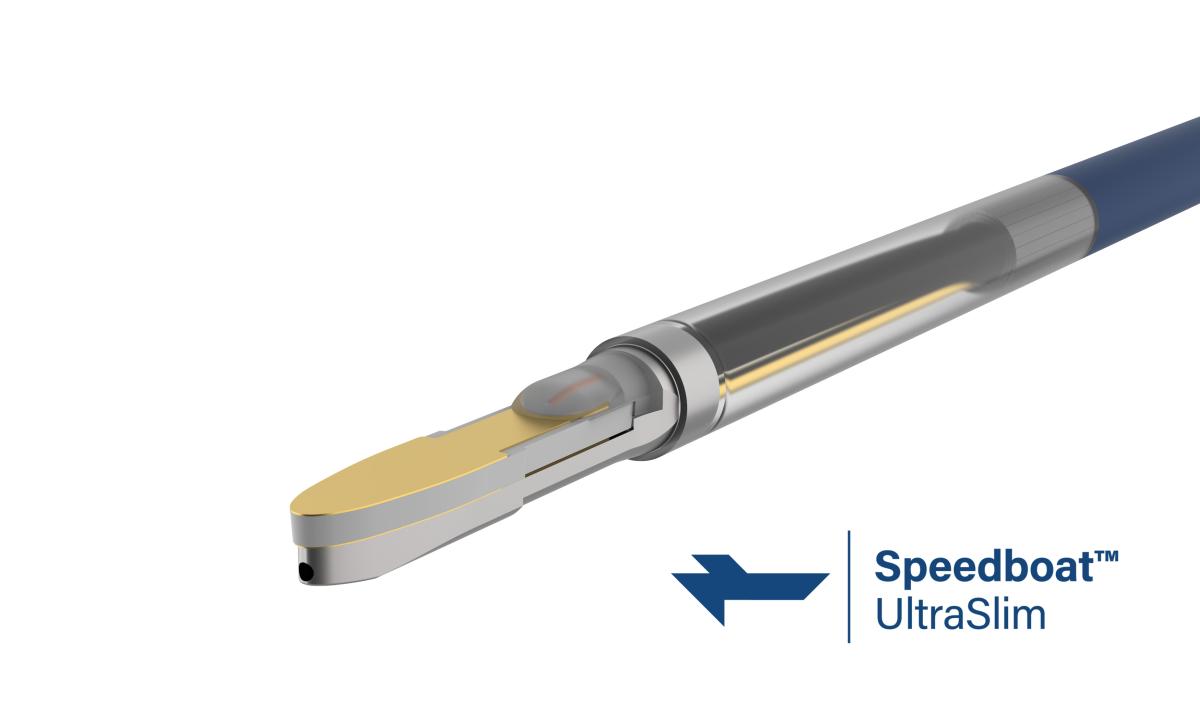
Speedboat® UltraSlim is the third device in Creo’s Speedboat® family of products. The device is the culmination of a long programme of work to miniaturise the technology to a scale which covers all the foreseeable market applications, resulting in blanket compatibility with all commercial endoscopes accessing the vast majority of GI endoscopic procedures and significantly increasing the opportunity for more clinicians and patients to benefit from Creo’s cutting-edge technology.
Speedboat® UltraSlim is targeting the therapeutic treatment of disease in the GI tract including cancer of the Bowel, Stomach and Oesophagus as well as surgical procedures to deal with abnormalities resulting in swallowing disorders and in some cases gastric reflux. Powered by Creo’s CROMA advanced energy platform, Speedboat® UltraSlim delivers advanced bipolar radiofrequency (“RF”) energy for controlled cutting and high frequency microwave (“MW”) energy for controlled coagulation of tissue in the GI tract.
Craig Gulliford, Chief Executive Officer of Creo Medical, said:
This is yet another landmark moment for Creo Medical. We know what a difference this even smaller device will make in terms of broadening access to advanced energy in endoscopy for a wider pool of clinicians. Speedboat UltraSlim is compatible with all major endoscopes, meaning clinicians can bring advanced energy to their practices, allowing more patients to be treated endoscopically, which avoids surgery and the associated secondary costs and risks. We will continue the early market release to a designated early adopter programme over the coming weeks before the full market launch in early 2024, with significant demand for this product already indicated from existing and potential Speedboat users.
Posted 13/12/2023
For press enquiries please contact media@creomedical.com. For all other enquiries please visit our Contact page.

